Clinical Efficacy
BB536 Improves Gastrointestinal Conditions
BB536 restores optimal bowel movements

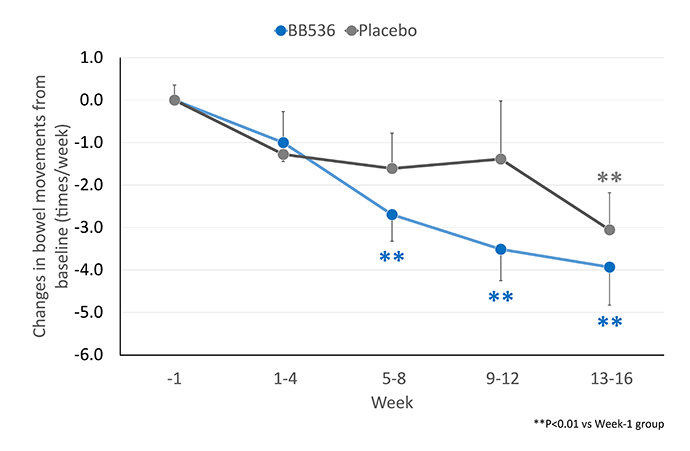
BB536 has long been recognized as one of the most effective probiotic strains for improvement of gastrointestinal conditions. Mounting clinical evidence have shown that consumption of dairy products containing BB536, including yogurts, yogurt drinks, and non-fermented milks, can improve the frequency of defecation and fecal characteristics in infants, children, adults, and elderly people with constipation or diarrhea[1-4].
In 168 patients aged >65 years who were receiving enteral tube feedings[4], consumption of BB536 (at both low and high doses of 25 billion and 50 billion CFU/day) for 16 weeks better improved the bowel movements of patients with infrequent defecation (≤4 times/week) as compared to the placebo control.
Administration of BB536 also restored regular bowel movements in patients with a high frequency of defecation (≥10 times/week). Intake of BB536 resulted in significantly higher prevalence of normally formed stools as compared to the placebo control.
Reference:
- 1Puccio, G., Cajozzo, C., Meli, F., Rochat, F., Grathwohl, D. and Steenhout, P., 2007. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition, 23(1), pp.1-8.
- 2Russo, M., Giugliano, F.P., Quitadamo, P., Mancusi, V., Miele, E. and Staiano, A., 2017. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Italian Journal of Pediatrics, 43(1), p.24.
- 3Wong, C.B., Odamaki, T. and Xiao, J.Z., 2019. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of Functional Foods, 54, pp.506-519.
- 4Kondo et al., 2013. Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World Journal of Gastroenterology: WJG, 19(14), p.2162.
BB536 Improves Intestinal Microenvironment
Enterotoxigenic Bacteroides fragilis (ETBF) is associated with colitis
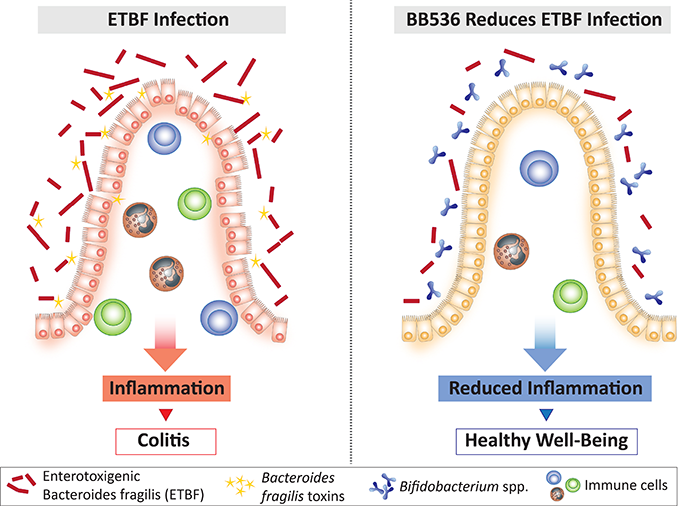
Accumulating evidence suggests that gut microbial dysbiosis may represent an etiological factor in the development of gastrointestinal diseases. In particular, it has been reported that certain bacterial species, such as the toxin-producing bacteria, enterotoxigenic Bacteroides fragilis (ETBF), are associated with acute and persistent diarrheal disease in patients with IBD[1] and the development of colorectal cancer[2,3].
BB536 eliminated ETBF in the gut
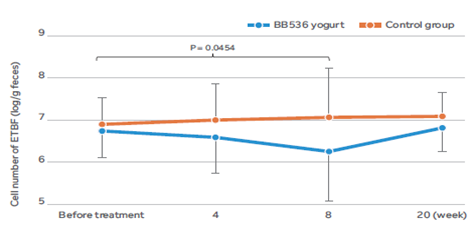
In 32 healthy adults who were the carriers of ETBF[4], ingestion of yogurt containing BB536 for 8 weeks had a discernible effect on the cell numbers of ETBF in the gut microbiota.
As a result, a significant decrease in the cell numbers of ETBF as compared to baseline values was observed in subjects receiving BB536 yogurt but not in the control milk group. However, when the subjects ceased the consumption of BB536 yogurt, the number of ETBF returned to the baseline level. These results suggest that continuous ingestion of BB536 could eliminate the opportunistic ETBF pathogens in the gut microbiota and condition the intestinal microenvironment.
Reference:
- 1Sears, C.L., 2009. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clinical Microbiology Reviews, 22(2), pp.349-369.
- 2Orberg, E.T., Fan, H., Tam, A.J., Dejea, C.M., Shields, C.D., Wu, S., Chung, L., Finard, B.B., Wu, X., Fathi, P. and Ganguly, S., 2017. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunology, 10(2), p.421.
- 3Wu, S., Rhee, K.J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H.R., Huso, D.L., Brancati, F.L., Wick, E., McAllister, F. and Housseau, F., 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Medicine, 15(9), p.1016.
- 4Odamaki, T., Sugahara, H., Yonezawa, S., Yaeshima, T., Iwatsuki, K., Tanabe, S., Tominaga, T., Togashi, H., Benno, Y. and Xiao, J.Z., 2012. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe, 18(1), pp.14-18.
BB536 Modulates Immune Response
BB536 reduces the incidence of influenza, probably by potentiating innate immunity
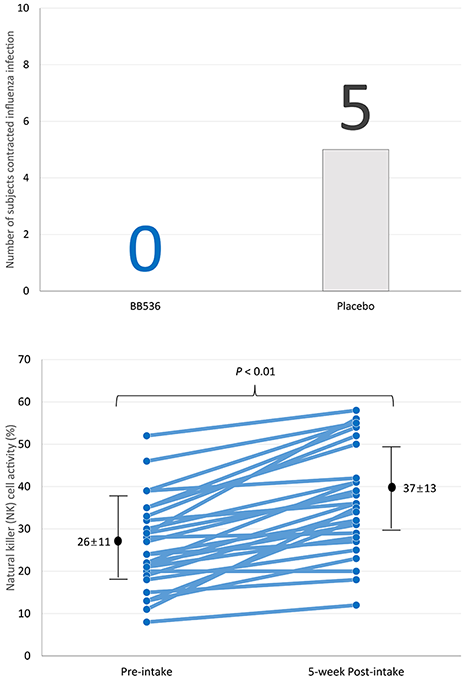
Multiple clinical studies have demonstrated that BB536 could modulate immune responses in human subjects, especially in the elderly[1]. It has been reported that cellular immune responses are weaker in the elderly and the decline in immune system function has been suggested to contribute to impaired vaccine efficacy and increased the risk of influenza virus infection. Importantly, BB536 was shown to be able to improve waning immunity in the elderly[2,3].
Twenty-seven elderly people aged 65 years and older were pre-administered with BB536 powder (100 billion CFU/day) for 5 weeks, during which they also received influenza vaccination at week 3. The subjects were then randomized into a BB536 group (n=13) and a placebo group (n=14) for 14 weeks. During the study period, the number of subjects with flu symptoms was fewer in those who consumed BB536 than those who did not. Also, natural killer cell activity and neutrophil bactericidal activity were increased after BB536 administration, providing evidence of enhanced immunity[3].
These results imply that BB536 may enhance the resistance of elderly consumers to pathogenic viruses and could be applied as a potential adjuvant to improve the immune response to influenza vaccines in the elderly.
Reference:
- 1Wong, C.B., Odamaki, T. and Xiao, J.Z., 2019. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of Functional Foods, 54, pp.506-519.
- 2Akatsu, H., Iwabuchi, N., Xiao, J.Z., Matsuyama, Z., Kurihara, R., Okuda, K., Yamamoto, T. and Maruyama, M., 2013. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. Journal of Parenteral and Enteral Nutrition, 37(5), pp.631-640.
- 3Namba, K., Hatano, M., Yaeshima, T., Takase, M. and Suzuki, K., 2010. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Bioscience, Biotechnology, and Biochemistry, 74(5), pp.939-945.
BB536 Alleviates Allergic Disorders
What is allergic rhinitis?
Allergic rhinitis, also known as hay fever, is a type of inflammation in the nose which is typically triggered by environmental allergens such as pollen, pet hair, dust, or mold. Signs and symptoms include a runny or stuffy nose, sneezing, red, itchy, and watery eyes, and swelling around the eyes. For instance, the seasonal allergic rhinitis caused by Japanese cedar pollen is one of the most prevalent forms of allergic disease in Japan and is considered a national affliction[1]. Japanese cedar pollinosis (JCPsis) is an immunoglobulin E (IgE)-mediated type I allergy triggered by exposure to the irritating cedar pollens.
Consumption of BB536 is protective against allergic rhinitis
Intakes of BB536 yogurt or lyophilized powder have been shown to potentially alleviate nasal and ocular allergic symptoms and modulate allergic immune response in people sensitive to Japanese cedar pollens[2-5].
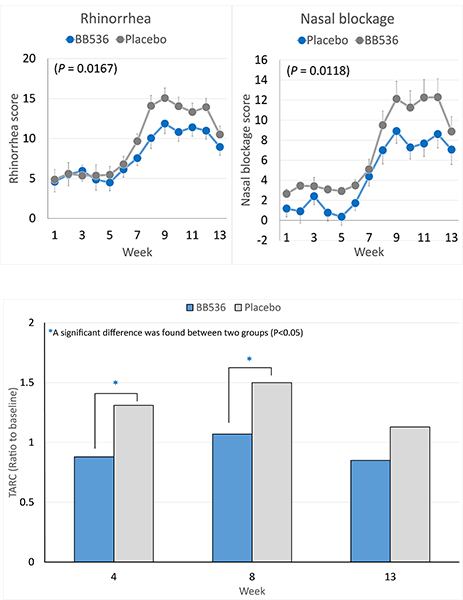
In 44 JCPsis subjects (aged 26-57 years)[3], consumption of BB536 powder (50 billion CFU/2g twice daily) for 13 weeks during the heaviest pollen season in 2005 significantly reduced the allergic scores of rhinorrhea and nasal blockage.
Intake of BB536 also significantly improved the T-helper 2 (Th-2)-skewed immune response that was occurred along with pollen dispersion. The plasma levels of thymus and activation-regulated chemokine (TARC) were remarkably normalized in subjects receiving BB536 powder than those of the placebo.
Reference:
- 1Yamada, T., Saito, H. and Fujieda, S., 2014. Present state of Japanese cedar pollinosis: the national affliction. Journal of Allergy and Clinical Immunology, 133(3), pp.632-639.
- 2Xiao, J.Z., 2006. Effect of Probiotic Bifidobacterium longum BBS36 in relieving clinical symptoms and modulating plasma cytokine levels in japanase cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial (vol 16, pg 86, 2006). Journal of Investigational Allergology and Clinical Immunology, 16(4), pp.273-273.
- 3Xiao, J.Z., Kondo, S., Yanagisawa, N., Takahashi, N., Odamaki, T., Iwabuchi, N., Miyaji, K., Iwatsuki, K., Togashi, H., Enomoto, K. and Enomoto, T., 2006. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clinical & Experimental Allergy, 36(11), pp.1425-1435.
- 4Xiao, J.Z., Kondo, S., Takahashi, N., Odamaki, T., Iwabuchi, N., Miyaji, K., Iwatsuki, K. and Enomoto, T., 2007. Changes in plasma TARC levels during Japanese cedar pollen season and relationships with symptom development. International Archives of Allergy and Immunology, 144(2), pp.123-127.
- 5Xiao, J.Z., Kondo, S., Yanagisawa, N., Miyaji, K., Enomoto, K., Sakoda, T., Iwatsuki, K. and Enomoto, T., 2007. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergology international, 56(1), pp.67-75.
