Clinical Efficacy
Protection Against Premature Birth Complications in Low Birth Weight Infants
Gut microbiota of premature infants
Prematurity, prolonged hospitalization, immunodeficiency, antibiotics use and delayed enteral feeding are challenging ways to begin life for preterm infants.
Premature infants often present with an immature gut and exhibit delayed gut colonization with beneficial commensal bacteria such as Bifidobacterium where instead they are more susceptible to colonization by Enterobacteriaceae and Enterococcus[1].
M-16V improves bifidobacterial colonization in preterm infants
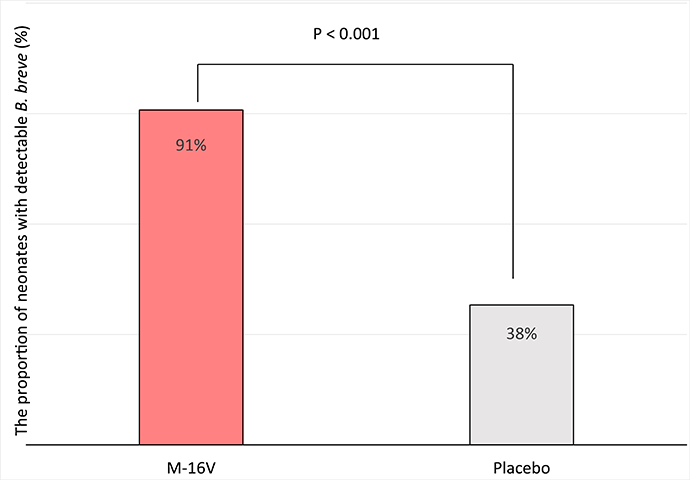
Supplementation with M-16V in preterm infants (gestation <33 weeks) and low birth weight infants (<2250 g) resulted in an earlier detection and longer maintenance of a bifidobacteria-dominant gut microbiome[2].
In 159 preterm neonates (gestation <33 weeks) ready to commence or on feeds for <12 h[3], consumption of M-16V (3 billion CFU/day) for three weeks significantly increased the levels of fecal B. breve as compared to placebo control where the B. breve counts were below detection level.
M-16V supplement was well-tolerated by all enrolled preterm neonates with no adverse effects including probiotic sepsis and deaths. The results suggest that M-16V is a suitable probiotic strain for routine use in preterm neonates to promote the acquisition of beneficial commensal bacteria.
Reference:
- 1Arboleya, S., Binetti, A., Salazar, N., Fernández, N., Solís, G., Hernandez-Barranco, A., Margolles, A., de los Reyes-Gavilan, C.G. and Gueimonde, M., 2012. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiology Ecology, 79(3), pp.763-772.
- 2Wong, C.B., Iwabuchi, N. and Xiao, J.Z., 2019. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients, 11(8), p.1724.
- 3Patole, S., Keil, A.D., Chang, A., Nathan, E., Doherty, D., Simmer, K., Esvaran, M. and Conway, P., 2014. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates-a randomised double blind placebo controlled trial. PloS one, 9(3), p.e89511.
Premature birth complications in low birth weight infants
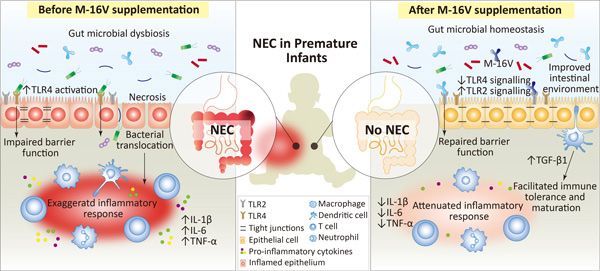
Premature infants are at elevated risk to develop multiple health comorbidities; one of which is the devastating necrotizing enterocolitis (NEC)[1].
Although the exact etiology and pathogenesis of NEC remain elusive, perturbation of the gut microbiota and a lack of bifidobacteria, leading to a hyperinflammatory response, appears to be a key factor that predisposes neonates to NEC[2].
M-16V may potentially prevent NEC, death and late onset sepsis in premature infants
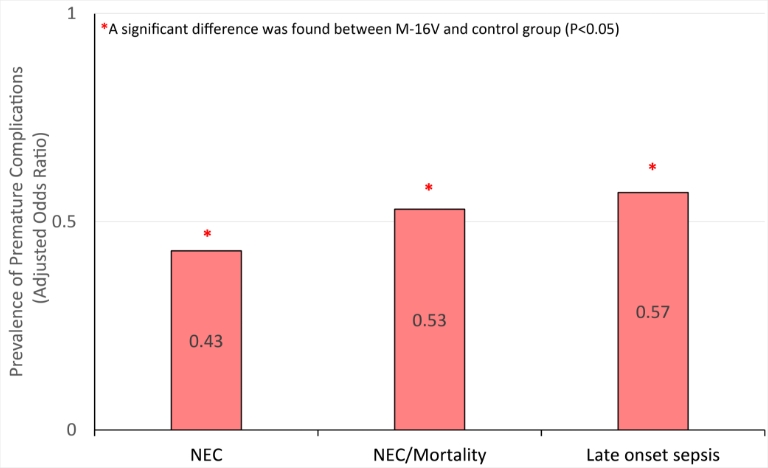
In a retrospective cohort study[3] involving 835 preterm neonates as historical control and 920 preterm neonates receiving M-16V supplementation (3 billion CFU/day) started when the infants were ready for enteral feeds and continued until the corrected age of 37 weeks, M-16V significantly lowered the incidence of "NEC Stage II", "NEC ≥ Stage II or all-cause mortality", late onset sepsis and age at full feeds.
These clinical findings underscore the potential roles of M-16V as a promising infant probiotic that could potentially impact the incidence, morbidity and mortality associated with NEC.
Reference:
- 1Berdon, W.E., Grossman, H., Baker, D.H., Mizrahi, A., Barlow, O. and Blanc, W.A., 1964. Necrotizing enterocolitis in the premature infant. Radiology, 83(5), pp.879-887.
- 2Groer, M.W., Luciano, A.A., Dishaw, L.J., Ashmeade, T.L., Miller, E. and Gilbert, J.A., 2014. Development of the preterm infant gut microbiome: a research priority. Microbiome, 2(1), p.38.
- 3Patole, S.K., Rao, S.C., Keil, A.D., Nathan, E.A., Doherty, D.A. and Simmer, K.N., 2016. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates-a retrospective cohort study. PloS one, 11(3), p.e0150775.
Reduction in the Prevalence of Atopic Dermatitis
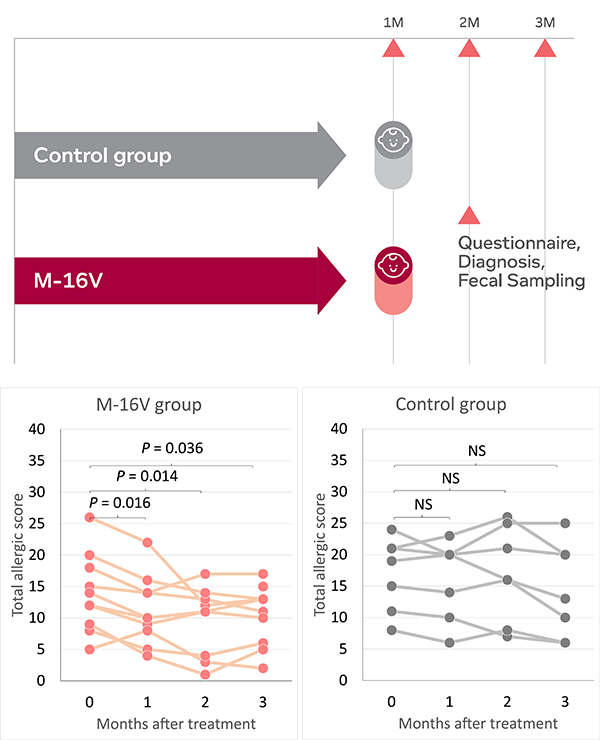
The prevalence of allergic diseases in infants has increased strikingly worldwide in the past few decades. While the pathogenesis of allergic diseases is likely to be multifactorial, deviations in gut colonization during early life are possible major factors promoting abnormal postnatal immune maturation[1]. Modulation of gut microbiota during early life through M-16V intervention has emerged as a potential measure to prevent allergic disorders in infants.
In 10 infants (aged 5.0 ± 18.5 months) with cow's milk allergies and atopic dermatitis who had a Bifidobacterium-deficit gut microbiota[2], administration of M-16V for one month significantly improved the symptoms of atopic dermatitis, increased the proportion of Bifidobacterium and decreased the levels of total aerobes in the gut microbiota of infants with atopic dermatitis.
- 1West, C.E., Jenmalm, M.C. and Prescott, S.L., 2015. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clinical & Experimental Allergy, 45(1), pp.43-53.
- 2Taniuchi S, Hattori K, Yamamoto A, Sasai M, Hatano Y, Kojima T, et al. Administration of Bifidobacterium to infants with atopic dermatitis: Changes in fecal microflora and clinical symptoms. J Applied Res. 2005;5: 387-396.
Prevention of Allergic Disorders
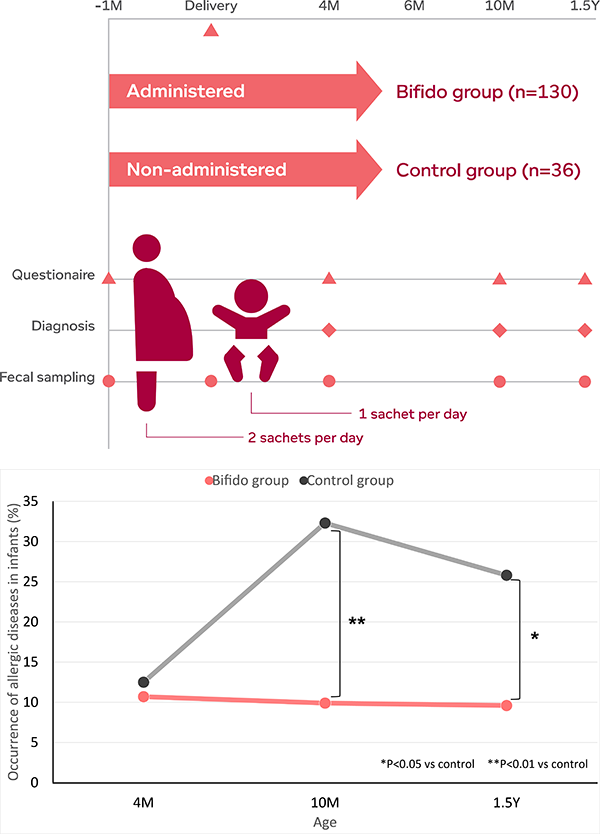
In an open trial[1], administration of M-16V in combination with BB536 during pregnancy as well as in postnatal period tied to lower the risk of developing allergic disorders in infants. The study involved 130 mothers who were provided with a daily powder formulation (two sachets daily, 1g/sachet) containing M-16V and BB536 (5 billition CFU/g of each strain) one month before the expected date of delivery and postnatally to their infants (one sachet daily) for six months. Another 36 mother-infant pairs who did not receive the bifidobacterial supplementation were served as the control.
Prenatal and postnatal supplementation with the bifidobacteria mixture significantly reduced the risk of developing eczema and atopic dermatitis in infants during the first 18 months of life as compared to the control group. A significant higher proportion of Bacteroidetes was observed in the microbiota of infants receiving the bifidobacteria mixture than that of the control group at four months of age. The relative abundance of Proteobacteria was also significantly lower in mothers receiving bifidobacteria mixture at the time of delivery than those in the control group, and was positively correlated with that of infants at four months of age.
These findings implicate that supplementation with bifidobacteria mixture of M-16V and BB536 during pregnancy may modulate both maternal and neonatal gut microbiota for prevention of allergies upset in infants later in life.
Reference:
- 1Enomoto, T., Sowa, M., Nishimori, K., Shimazu, S., Yoshida, A., Yamada, K., ... & Odamaki, T. (2014). Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergology International, 63(4), 575-585.
